Radiotherapy quality control in clinical trials

The role of radiotherapy in oncology
Watch the full version of our July 07 webinar on this topic.
Simply register to view it.
In 2018, there were over 18 million new cases of cancer worldwide. Forecasts show that this number will unfortunately continue to rise, reaching 27.5 million cases by 2040 i.

Cancer treatment is multidisciplinary, mainly combining surgery, chemotherapy and radiotherapy. Radiotherapy remains the main treatment, used for over 50% of patients.
However, according to the study "Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years" ii published in 2018, interventional radiotherapy studies accounted for just 5.3% of oncology trials over the period 2006-2016. One of the explanations for this is the greater difficulty in funding these studies, which are nevertheless necessary to evaluate new radiotherapy techniques.
However, according to "Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy" iii published in 2018, the place of radiotherapy is more important in immunotherapy trials. The combination of radiotherapy and immunotherapy in studies has been increasing in recent years, and this is likely to continue in the coming years.
Finally, if we look at all the oncology studies published on ClinicalTrial.gov, nearly 25% of them include radiotherapy, whether or not it is the primary treatment objective. Although ClinicalTrial.gov is estimated to reference only 70% of studies, this proportion seems realistic.
Key figures:
- Radiotherapy is used to treat over 50% of patients.
- Radiotherapy studies account for only 5.3% of oncology trials.
- Radiotherapy is found in around 25% of Oncology clinical trials.
Radiotherapy today
Radiotherapy has undergone major changes in recent years, in particular to improve tumor targeting and monitoring, and to better adapt patient treatment. Most machines now incorporate on-board imaging, such as MRI and CT scans, to ensure that the planned treatment is actually carried out.
Nevertheless, radiotherapy remains a complex treatment requiring a number of different stages:
- Imaging: to define where to treat, making increasing use of multimodality imaging
- Planning: how to treat the tumor while avoiding organs at risk (OARs)
- Treatment: to deliver what has been planned over the course of all sessions.
This complexity of radiotherapy management will be more difficult to harmonize in studies, and the problems to be solved lie mainly in inter-operator and inter-center variability:
- Compliance with contouring recommendations: are the tumor and organs at risk defined and contoured in the same way in all centers?
- Compliance with dosimetric constraints: are all centers treating according to the same protocol and with the same dosimetric constraints?
Without the harmonization and standardization of treatments between different patients, the results obtained cannot be properly assessed and analyzed. This is one of the main aims of the report put forward by ICRU in 2013. iv.
The impact of protocol deviations in clinical trials
In 2013, the study "Radiotherapy Protocol Deviations and Clinical Outcomes: A Meta-analysis of Cooperative Group Clinical Trials" v shows the impact of protocol deviations on study quality and outcome. This analysis was carried out on 8 studies conducted by recognized cooperative groups. In this publication, protocol deviations were observed in 8 to 71% of cases (median 32%). These deviations had a significant impact on the results observed, both in terms of patient survival and the study's secondary objectives.
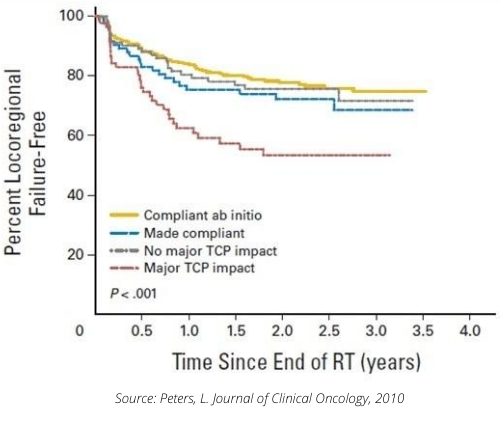
In particular, the 2010 study "Critical Impact of Radiotherapy Protocol Compliance and Quality in the Treatment of Advanced Head and Neck Cancer: Results From TROG 02.02" vi assessed the impact of treatment quality in a Phase III ENT study involving 861 patients at 82 centers in 16 countries. This study already had quality control and a centralized review of treatment plans. A posteriori, there was a centralized review of the plans delivered to all patients, with the 612 patients considered "protocol compliant", assessed mainly in terms of dosimetry, on one side, and the 208 "non-compliant" patients on the other. Among the latter patients, an independent expert was tasked with separating them into two groups: those for whom deviation from protocol had an estimated impact on treatment, and those for whom deviation would have no impact.
The results show very different survival and recurrence curves between the groups. We can see that patients classified as major deviations have a different survival curve to other patients.
It was also noted that the response to the study would have been different if the results had been compared between patients who had received "Good radiotherapy" and those who had had "Bad radiotherapy".
The main need expressed in this study was for real-time quality control to be performed prospectively, prior to patient treatment.
The subject remains topical, particularly with the 2018 study "Quality of radiotherapy reporting in randomized controlled trials of prostate cancer" vii.This reviews 59 prostate trials from 1987 to 2016. The authors analyzed 10 criteria they considered important for treatment quality. The study was considered good if 7 criteria were met. Only 40% of trials met these criteria.
In another ENT study from 2019, "The impact of clinical trial quality assurance on outcome in head and neck radiotherapy treatment" viii, the authors defined quality indexes for target volume contouring, organ-at-risk contouring and dosimetric coverage. They then created a score called the "RTQA score", which they analyzed in relation to clinical results. This study also showed an impact on the different survival curves of patients with a poor RTQA score.
These different studies clearly demonstrate the importance and benefits of rigorous quality control of radiotherapy in clinical oncology studie
Of course, this quality control comes at a cost, mainly in terms of the human resources required to review the files, but it is necessary for the quality of treatments and the relevance of the results obtained in studiesix. For this reason, many journals will no longer accept a manuscript if the quality control methodology for radiotherapy has not been described and documented.
“Although the incorporation of an RT-QA program may be a costly initial component of clinical trials, in the long term we cannot afford to run trials dependent on high-quality RT without it.”
Source: McDowell L., IJROBP, 2018
The solutions to be implemented are well known
Radiation Therapy Quality Assurance (RTQA). Numerous recommendations have been published, such as those of the AAPM x or the Global Harmonisation Group xi, which brings together learned societies and cooperative groups at international level, and recommends in particular :
- Dummy Runs, i.e. contouring and dosimetry exercises, or even recalibration on a fictitious clinical case, to ensure that each investigating center has fully integrated the study's treatment protocol.
- Once the center has been "approved" on the basis of the Dummy Runs, they recommend a centralized review of the plans prior to treatment of all patients, the first patients from each center or on randomly selected patients.
AQUILAB solutions for RT QA in clinical trials
AQUILAB has been involved for many years in improving the quality and harmonization of radiotherapy in clinical trials. Our expertise and technological solutions make it easier for sponsors to implement quality assurance protocols.
In particular, our Onco Place solution is a web-based platform that enables :
- Manage clinical trial participants. There is an investigator area, a sponsor area and an expert area. These three areas are distinct, but connected to each other to coordinate the workflow between the different participants.
- Anonymize, structure and centralize DICOM / DICOM RT data (images, structures, plans and doses) required for quality control.
- Analyze images, contours and treatment plans from all TPS on the market, using the review tools in our ARTIVIEW solution.
Depending on the study protocol, each project can be customized to ensure appropriate follow-up of inclusions, while guaranteeing the security of the information exchanged.
You can find a demonstration of the Onco Place platform in the second part of our webinar on centralized quality control of radiotherapy in clinical trials.
Bibliography
i Global Cancer Observatory. https://gco.iarc.fr/
ii Liu, X., Zhang, Y., Tang, L. L., Le, Q. T., Chua, M. L. K., Wee, J. T. S., … Ma, J. (2018).
Characteristics of radiotherapy trials compared with other oncological clinical trials in the past 10 years.
JAMA Oncology, 4(8), 1073–1079. https://doi.org/10.1001/jamaoncol.2018.0887
iii Cushman, T. R., Caetano, M. S., Welsh, J. W., & Verma, V. (2018).
Overview of ongoing clinical trials investigating combined radiotherapy and immunotherapy.
Immunotherapy, 10(10), 851–859. https://doi.org/10.2217/imt-2018-0019
iv Grégoire, V., & Mackie, T. R. (2011).
State of the art on dose prescription, reporting and recording in Intensity-Modulated Radiation Therapy (ICRU report No. 83).
Cancer/Radiotherapie, 15(6–7), 555–559. https://doi.org/10.1016/j.canrad.2011.04.003
v Ohri, N., Shen, X., Dicker, A. P., Doyle, L. A., Harrison, A. S., & Showalter, T. N. (2013).
Radiotherapy Protocol Deviations and Clinical Outcomes: A Meta-analysis of Cooperative Group Clinical Trials.
JNCI Journal of the National Cancer Institute, 105(6), 387–393. https://doi.org/10.1093/jnci/djt001
vi Peters, L. J., O’Sullivan, B., Giralt, J., Fitzgerald, T. J., Trotti, A., … Rischin, D. (2010).
Critical Impact of Radiotherapy Protocol Compliance and Quality in the Treatment of Advanced Head and Neck Cancer: Results From TROG 02.02.
Journal of Clinical Oncology, 28(18), 2996–3001. https://doi.org/10.1200/JCO.2009.27.4498
vii Soon, Y. Y., Chen, D., Tan, T. H., & Tey, J. (2018).
Quality of radiotherapy reporting in randomized controlled trials of prostate cancer.
Radiation Oncology, 13(1), 1–8. https://doi.org/10.1186/s13014-018-1053-7
viii Zhong, H., Men, K., Wang, J., van Soest, J., Rosenthal, D., Dekker, A., … Xiao, Y. (2019).
The impact of clinical trial quality assurance on outcome in head and neck radiotherapy treatment.
Frontiers in Oncology, 9(AUG), 1–7. https://doi.org/10.3389/fonc.2019.00792
ix McDowell, L. J., & Corry, J. (2018).
A Call to Arms: Radiation Therapy Quality Assurance in the Next Generation of Clinical Trials.
International Journal of Radiation Oncology Biology Physics, 102(5), 1590–1591. https://doi.org/10.1016/j.ijrobp.2018.07.2001
x AAPM Report 113, T. G. (2018).
Guidance for the Physics Aspects of Clinical Trials. https://doi.org/10.37206/172
xi Melidis, C., Bosch, W. R., Izewska, J., Fidarova, E., Zubizarreta, E., … Hurkmans, C. W. (2014)
Radiation therapy quality assurance in clinical trials – Global harmonisation group.
Radiotherapy and Oncology, 111(3), 327–329. https://doi.org/10.1016/j.radonc.2014.03.023